Minimizing Risk. Every Procedure. Every Time.™
For more than 40 years, Epimed has produced high quality and effective needles and catheters for Pain Medicine and Regional Anesthesia procedures.
Featured Products
RF™ Probe Cable Marker Set
 RF™ Probe Cable Marker Sets come with 4 color-coded cable identification ...view more
RF™ Probe Cable Marker Sets come with 4 color-coded cable identification ...view moreG-21 Epidural Catheter™
 G-21 Epidural Catheters™ are smaller than standard gauge-sized epidural c...view more
G-21 Epidural Catheters™ are smaller than standard gauge-sized epidural c...view moreCobra RF™
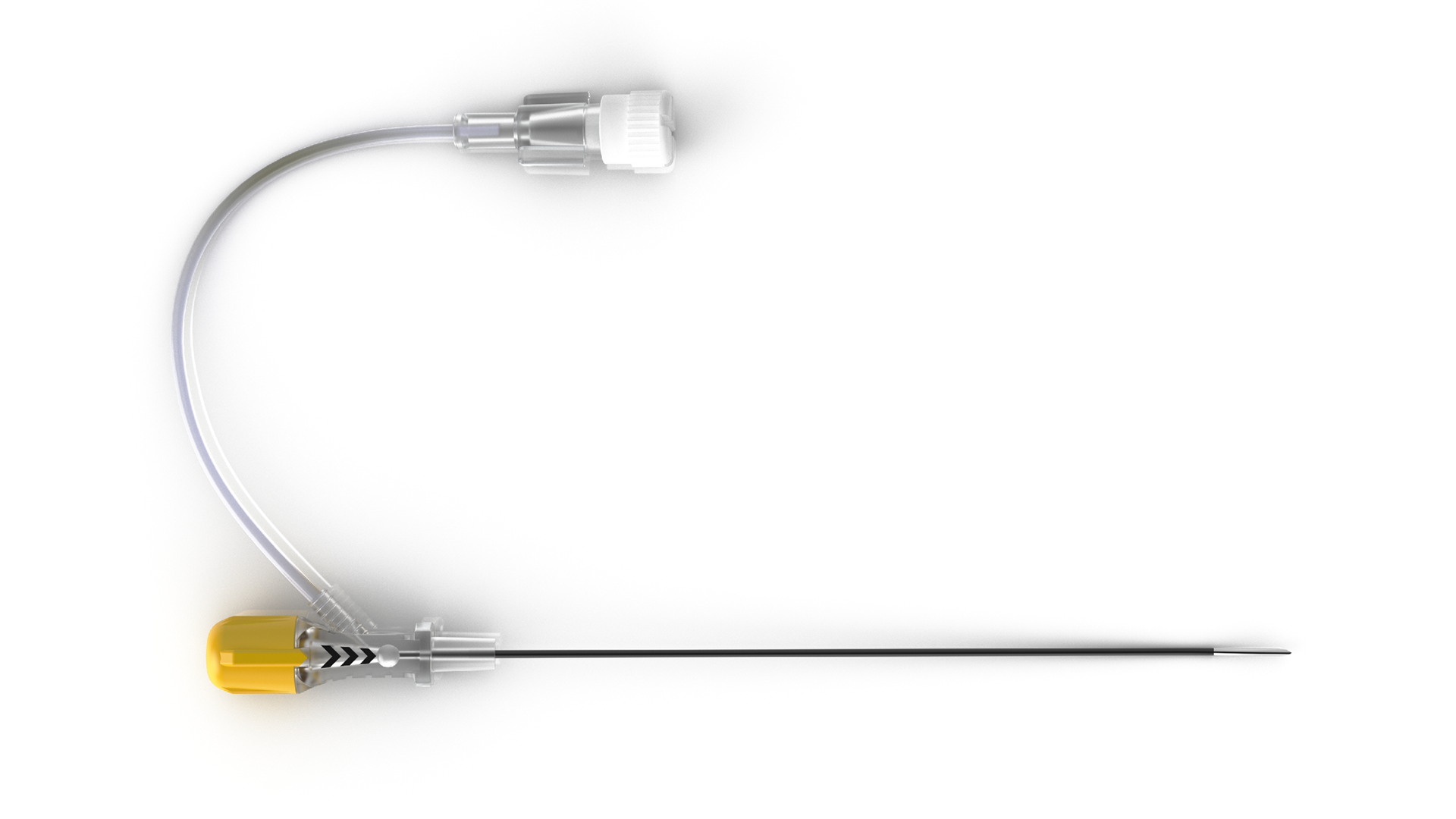 The Cobra RF™ Cannula conveniently eliminates the need to switch between ...view more
The Cobra RF™ Cannula conveniently eliminates the need to switch between ...view moreAccura-C™
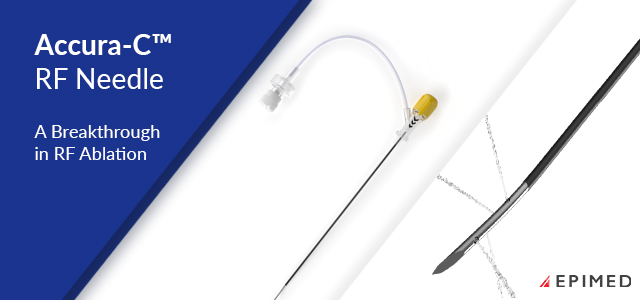
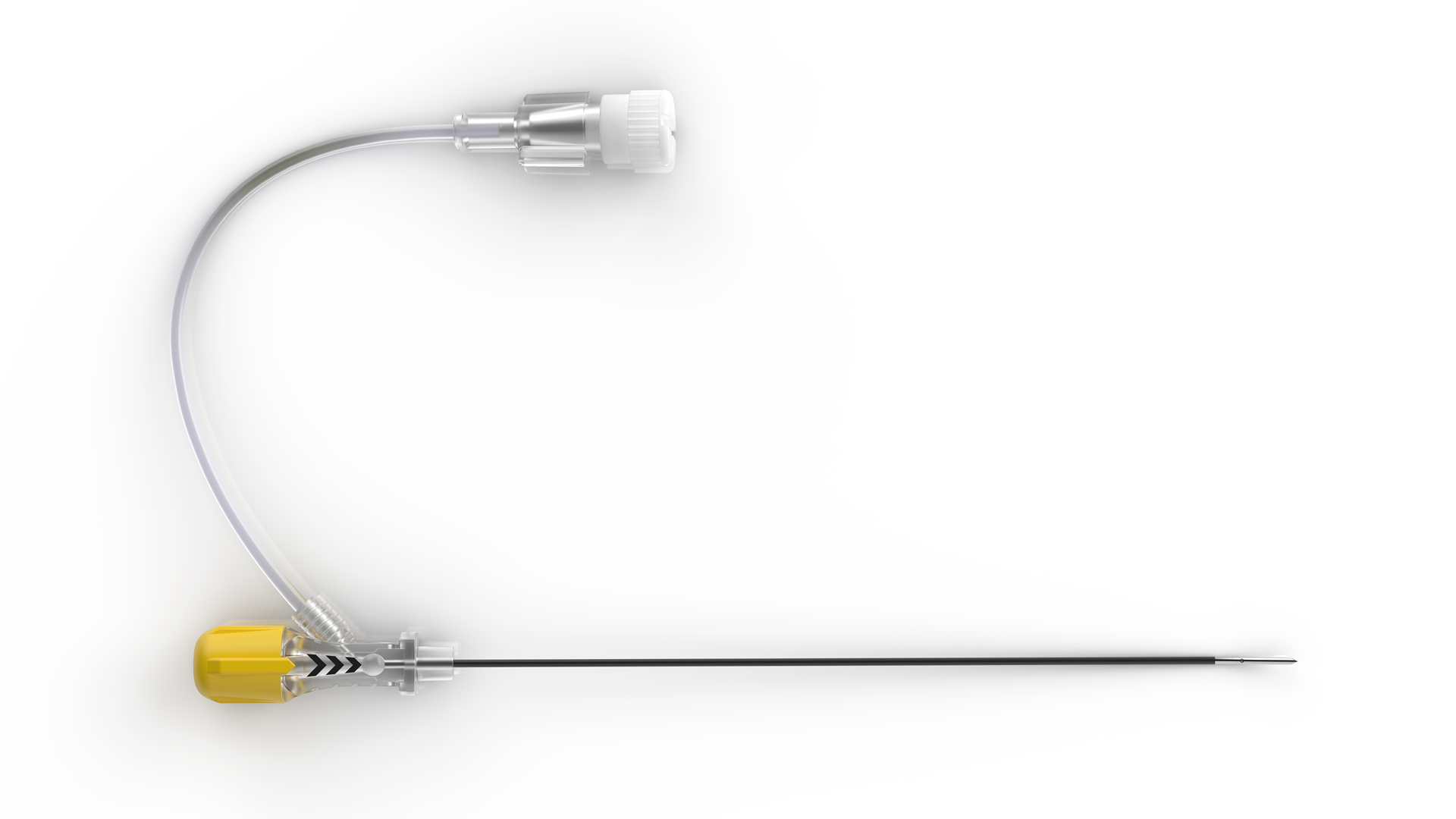 Accura-C™ larger lesion radiofrequency needles feature dual-injection hub...view more
Accura-C™ larger lesion radiofrequency needles feature dual-injection hub...view moreView All 9
RF™ Sharp Needles
 RF™ Sharp Needles are disposable, straight and Coudé®, sharp radiofrequ...view more
RF™ Sharp Needles are disposable, straight and Coudé®, sharp radiofrequ...view moreCobra RF™
 The Cobra RF™ Cannula conveniently eliminates the need to switch between ...view more
The Cobra RF™ Cannula conveniently eliminates the need to switch between ...view moreRF™ Grounding Pad
 RF™ Grounding Pads are single-use, non-sterile, latex-free, individually ...view more
RF™ Grounding Pads are single-use, non-sterile, latex-free, individually ...view moreNitinol Hyperflex™ RF™ Probes
 Epimed Reusable Nitinol Hyperflex™ RF™ Probes feature a lightweight hub...view more
Epimed Reusable Nitinol Hyperflex™ RF™ Probes feature a lightweight hub...view moreView All 9
RF-A™ Sharp Needles
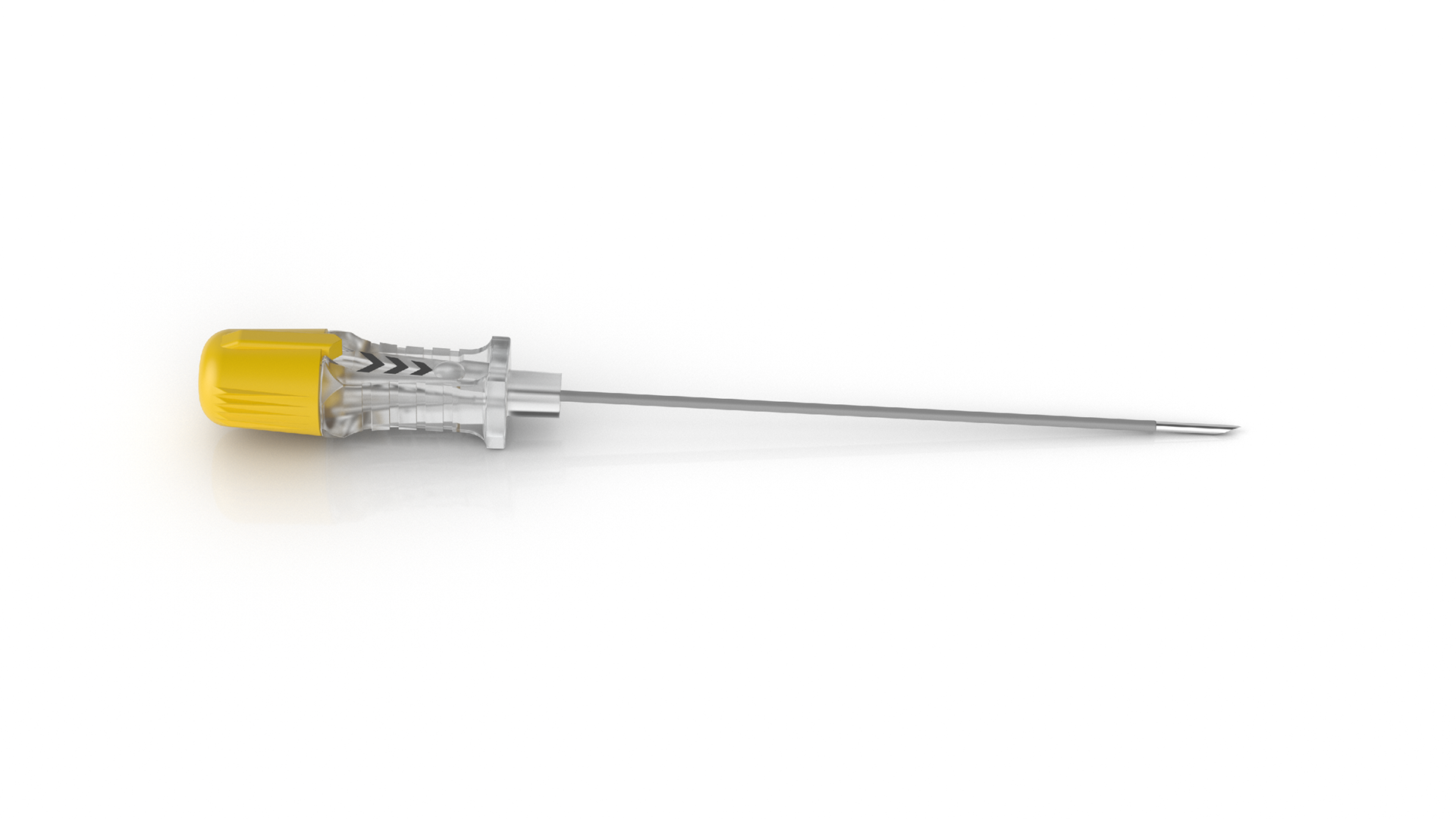 RF-A™ Sharp Needles are sharp ablation needles designed for pain manageme...view more
RF-A™ Sharp Needles are sharp ablation needles designed for pain manageme...view moreBlunt Access Cannula (BAC)
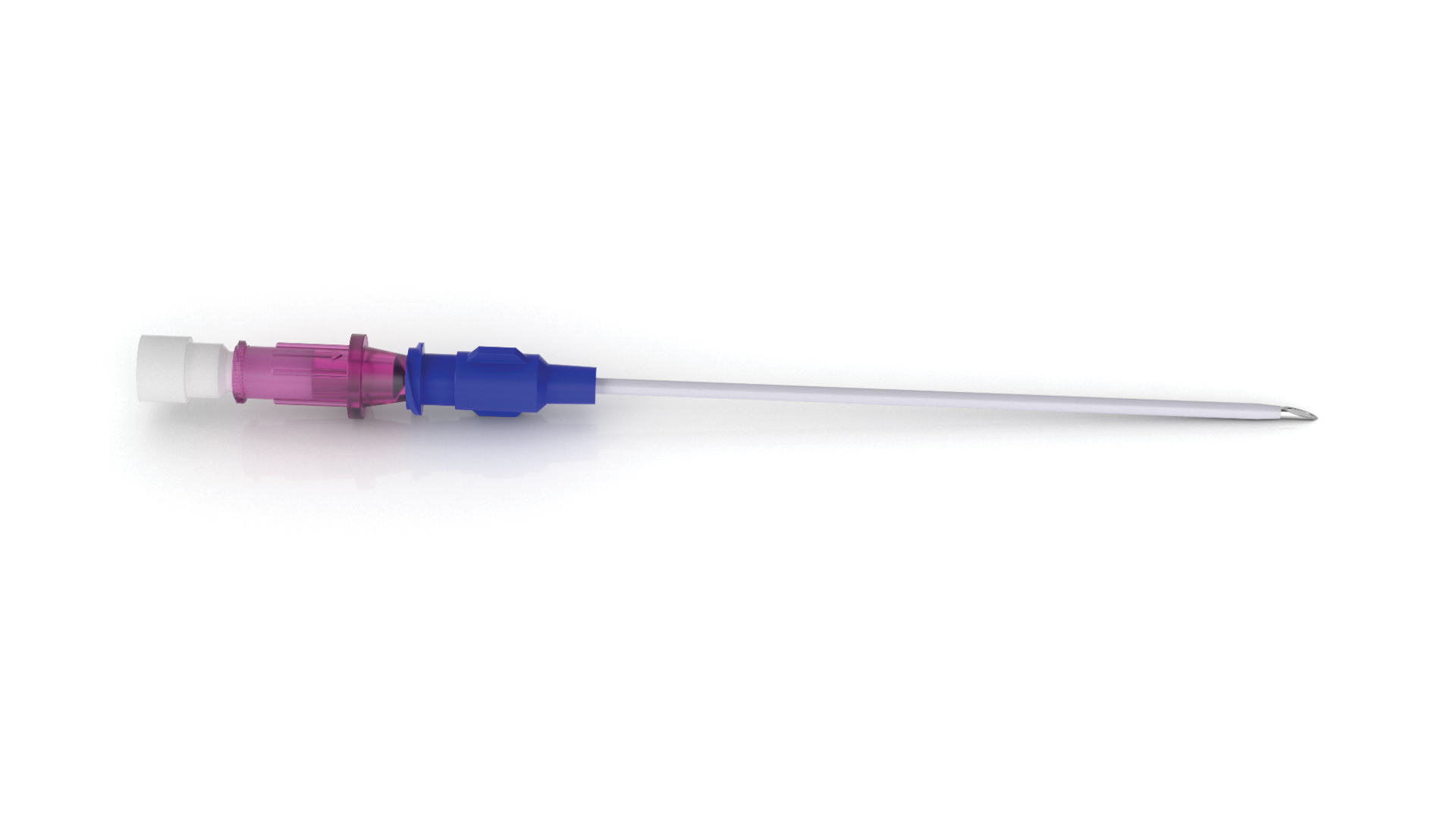 Blunt Access Cannulas (BAC) are introducer needles for blunt nerve block ne...view more
Blunt Access Cannulas (BAC) are introducer needles for blunt nerve block ne...view moreAccura-C™
 Accura-C™ larger lesion radiofrequency needles feature dual-injection hub...view more
Accura-C™ larger lesion radiofrequency needles feature dual-injection hub...view moreDisposable RF™ Probes
 Epimed Disposable RF™ Probes feature a lightweight hub, breakage-resistan...view more
Epimed Disposable RF™ Probes feature a lightweight hub, breakage-resistan...view moreView All 9
Essential Epidural Needles
 Epimed Essential Epidural Needles with Tuohy tip are injection-only needles...view more
Epimed Essential Epidural Needles with Tuohy tip are injection-only needles...view moreStingray® Connector
 Our award winning, patented Stingray® Connector is designed to make connec...view more
Our award winning, patented Stingray® Connector is designed to make connec...view moreTuohy Single Shot Kit
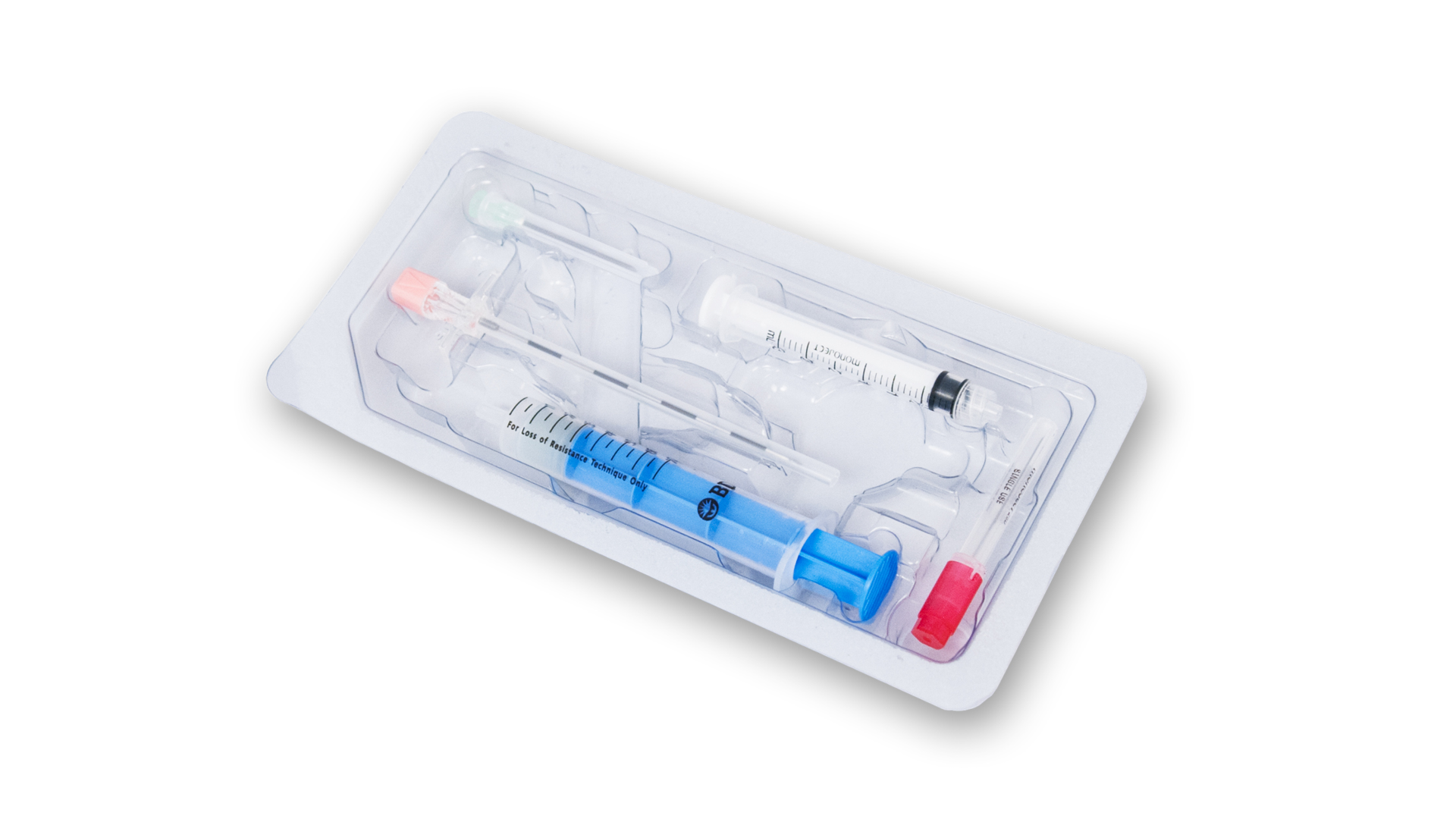 Tuohy Single Shot Kits with Plastic LOR are Epimed's epidural injection nee...view more
Tuohy Single Shot Kits with Plastic LOR are Epimed's epidural injection nee...view moreGrounding Pad Cable
 Reusable Grounding Pad Cables are radiofrequency plate cables for use with ...view more
Reusable Grounding Pad Cables are radiofrequency plate cables for use with ...view moreView All 9
Flex Vest and Skirt X-Ray Apron
 Flex Vest and Skirt X-Ray Radiation Protection Aprons with thyroid shields ...view more
Flex Vest and Skirt X-Ray Radiation Protection Aprons with thyroid shields ...view moreFlex X-Ray Apron
 Flex X-Ray Radiation Protection Aprons come with thyroid shields and should...view more
Flex X-Ray Radiation Protection Aprons come with thyroid shields and should...view moreXR-SecureTouch
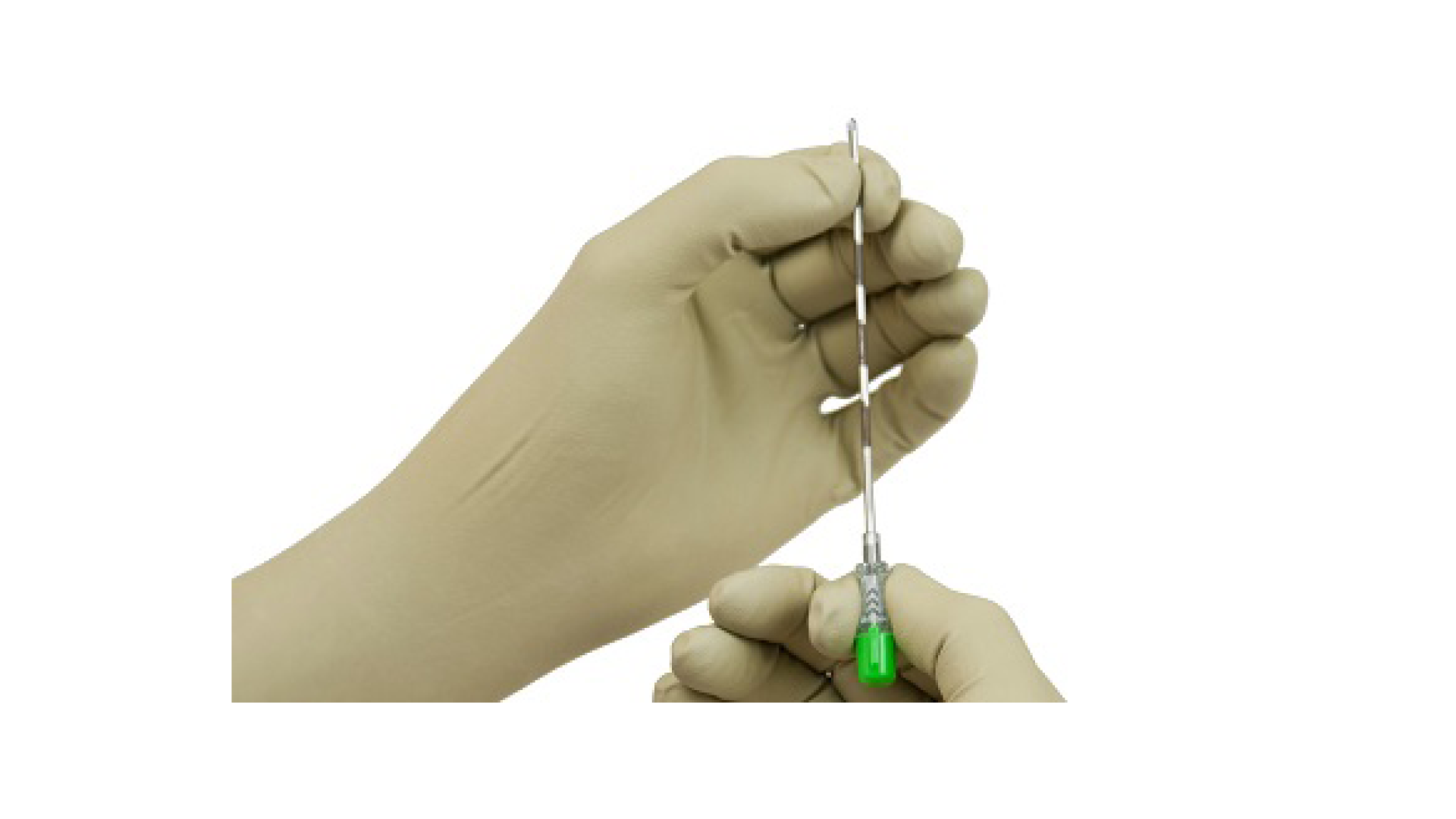 XR1 Features: High Tensile Strength, Textured Finger/Palm, Lead-Free and No...view more
XR1 Features: High Tensile Strength, Textured Finger/Palm, Lead-Free and No...view moreREADY-TO-GO Apron
 READY-TO-GO Radiation Protection Aprons with magnetic thyroid shields come ...view more
READY-TO-GO Radiation Protection Aprons with magnetic thyroid shields come ...view moreView All 9
Grounding Pad Cable
 Reusable Grounding Pad Cables are radiofrequency plate cables for use with ...view more
Reusable Grounding Pad Cables are radiofrequency plate cables for use with ...view moreStandard Extension Set
 Epimed Standard Extension Sets for microbore injections come with standard ...view more
Epimed Standard Extension Sets for microbore injections come with standard ...view moreDouble Nelson Extension Set
 Double Nelson Injection Extension Sets contain of microbore tubing with dis...view more
Double Nelson Injection Extension Sets contain of microbore tubing with dis...view moreRF™ Probe Sterilization Tray
 RF™ Probe Sterilization Trays for autoclave use can secure up to four rad...view more
RF™ Probe Sterilization Trays for autoclave use can secure up to four rad...view moreView All 9
Epimed Quality
Our reputation in Interventional Pain Medicine and Regional Anesthesia is rooted in a commitment to quality. We focus on providing our customers with products that exceed expectations and deliver consistent results every procedure, every time.
%
Customer Satisfaction
Years of Experience

The Business Side of Practicing Medicine
Epimed’s Physician Services Department helps fellows and veteran doctors succeed when navigating the complicated business side of practicing pain medicine. Topics include reimbursement, negotiating contracts, marketing, billing and coding, and more.
Bringing Your Idea to Life
Epimed helps you develop and adapt new and existing products. We are an original equipment manufacturer that offers a multitude of customized services to fit your needs.